MS Validated Antibodies (MSVA) - Monoclonal IHC antibodies
Large-Scale Validated Antibodies for Immunohistochemistry of normal & cancer tissue
MS Validated Antibodies (MSVA) is specialized in immunohistochemistry and provides a unique wealth of information for every antibody on the MSVA homepage, because the key to successful Immunohistochemistry is: Know your antibody!
Most of MSVA’s antibodies are rabbit recombinant antibodies. These share the advantage of complete reproducibility of their production because they are not generated by potentially unstable hybridoma cells but produced “in lab” based on the known DNA sequence of the antibody chains.
Key benefits of MSVA
- Extremely sophisticated product selection – only 130 antibodies out of 4000 candidates were selected for the MSVA portfolio
- Extensive validation process on tissues
- Unique documentation
- A gallery for normal and tumor tissues (> 50 images) is supplied for each antibody
- Only 1 antibody per target to avoid confusion on what clone to select
- Protocol suggestions (Manual Protocol, Dako, Leica & Ventana autostainers)
- Published data on the performance characteristics of MSVA antibodies on >10,000 tumors are compiled on the MSVA website
E.g. available for: PLAP, CPA1, Mesothelin, CK19, CK6, MUC5AC, Arginase-1, DOG-1
Antibody validation is different for antibodies in immunohistochemistry
Antibodies intended for use on formalin fixed tissues must be specifically validated on formalin fixed tissues. According to the International Working Group for Antibody Validation (IWGAV), there are only two ways how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues:
- Orthogonal strategy: Comparison with a second independent method for target expression measurement across a large number of different tissue types
- Independent antibody strategy: Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also seen with other antibodies for the same target
The MSVA Validation Approach: "orthogonal validation” + “independent antibody validation”
1. Orthogonal Strategy - Look up & Stain
RNA data and protein are looked up in the Human Protein Atlas (HPA) to see in which tissues staining is expected. Data look up includes the normal tissue expression RNA databases “the Human Protein Atlas (HPA) RNA-seq tissue dataset”, the FANTOM5 project, and the Genotype-Tissue Expression (GTEx) project.
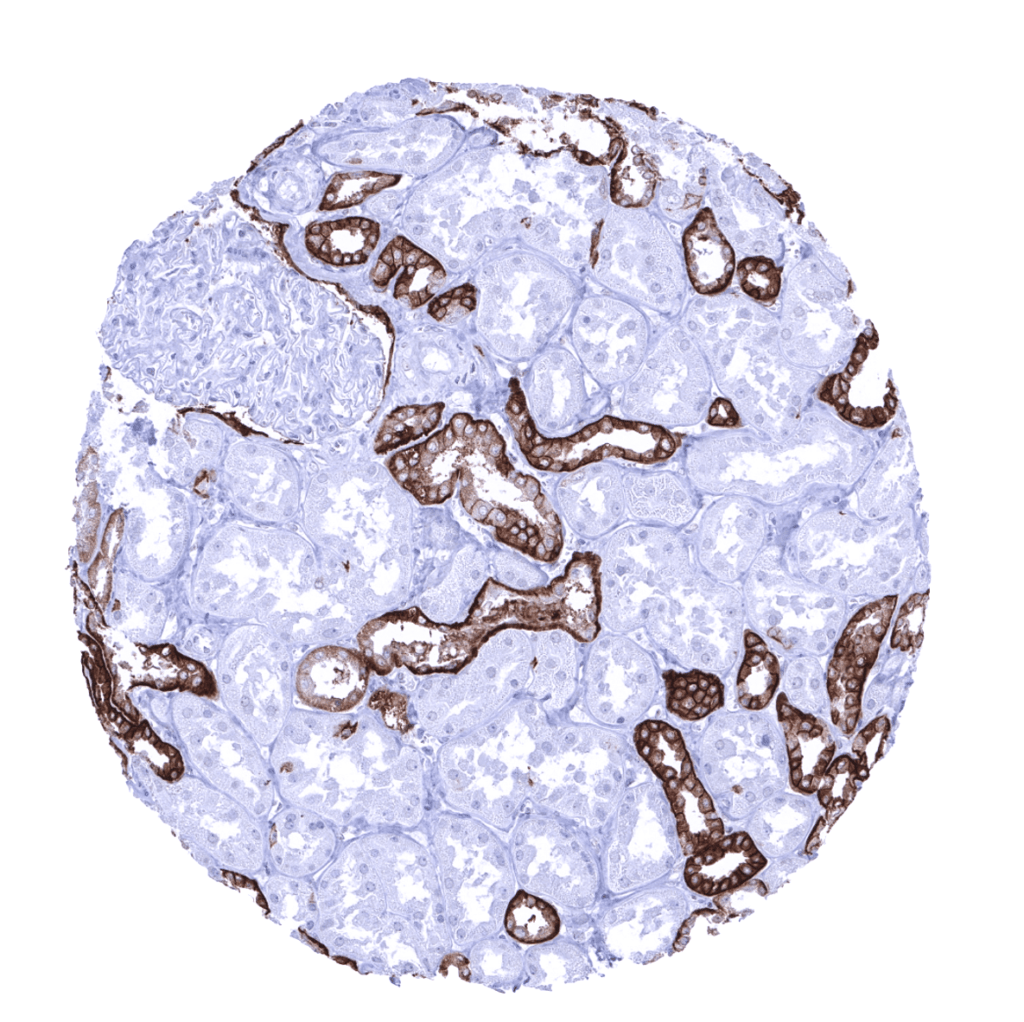
Staining with the antibody on a tissue microarray with different normal tissues and different tumor types. Check if the staining is consistent with the HPA data.
2. Independent antibody validation - Compare
Comparison with independent clones for the same target – including established commercial (IVD/IVDR) antibodies when possible – to confirm or disconfirm staining.
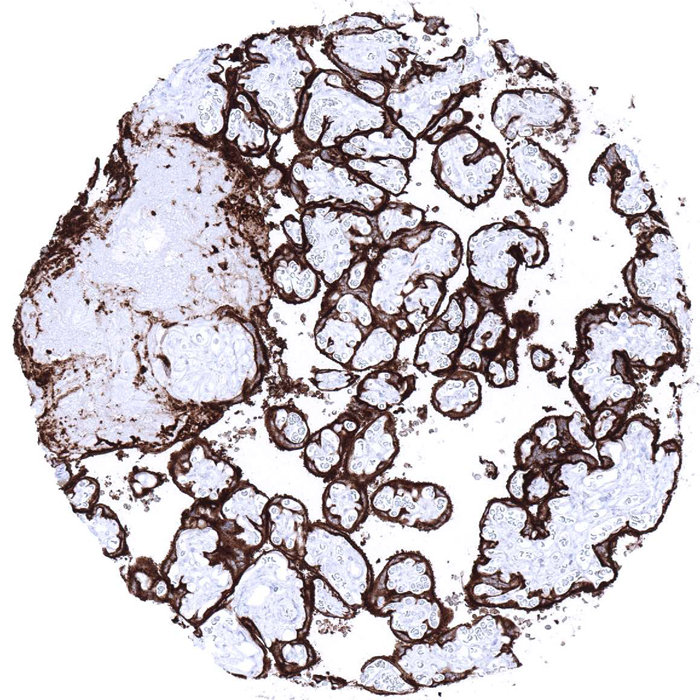
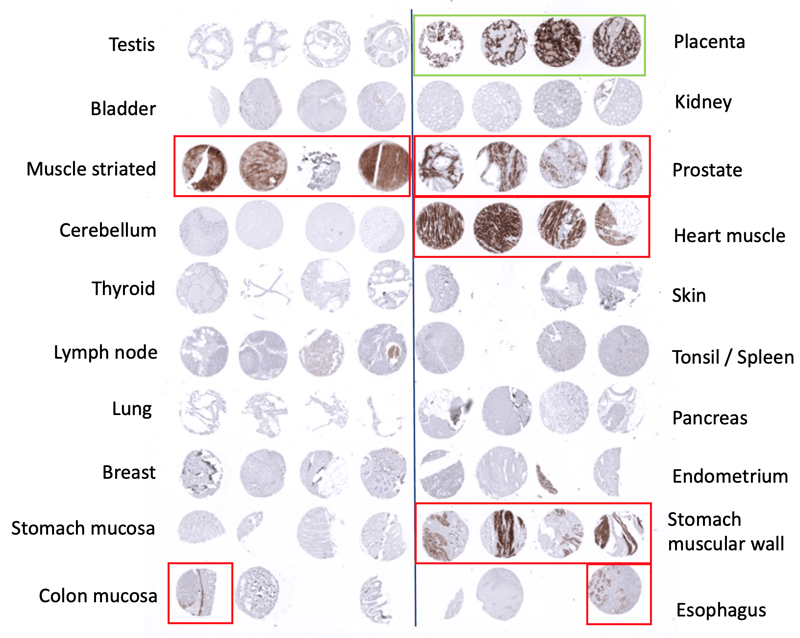
For the given example (PLAP MSVA candidate #2) the placenta staining is confirmed. But other positive stainings are obviously non-specific, as the other antibody does not show them and RNA expression does not suggest these stainings.
We compare with an established clone (8A9) to confirm the results (see image beside). Again: Placenta staining is confirmed. And even more non-specificity for this PLAP antibody!
3. Decision
For the given example: PLAP antibody „MSVA Product candidate #2” – discarded due to cross-reactivity“

Antibody quality is key for multiplex fluorescence immunohistochemistry (mfIHC)
Multiplex fluorescence immunohistochemistry (mfIHC) allows the application of multiple antibodies to one tissue slide. Sophisticated analysis frameworks utilize artificial intelligence (AI) for specific cell type identification defined by a combination of positive and negative antibody stainings as well as the spatial relationship between these cell types.
Because of the high number of different “colors” on each stained slide and impaired morphologic cell type distinction in fluorescence imaging, visual quality control of individual antibodies becomes impossible. However, non-specific or cross-reactive antibody staining of one or several antibodies used in a multicolor panel can severely contaminate the data and conclusions drawn from experiments.
Therefore, high-quality antibodies resulting in minimal non-specific binding (signal to noise ratio) and a demonstarted & documented lack of cross-reactivity are critical for experimental success. Hence, the quality of the antibodies assembled in a mfIHC panel is pivotal!
Examples of mfIHC images containing a variable number of antibodies
(Images: Courtesy of Dr. Niclas C. Blessin)
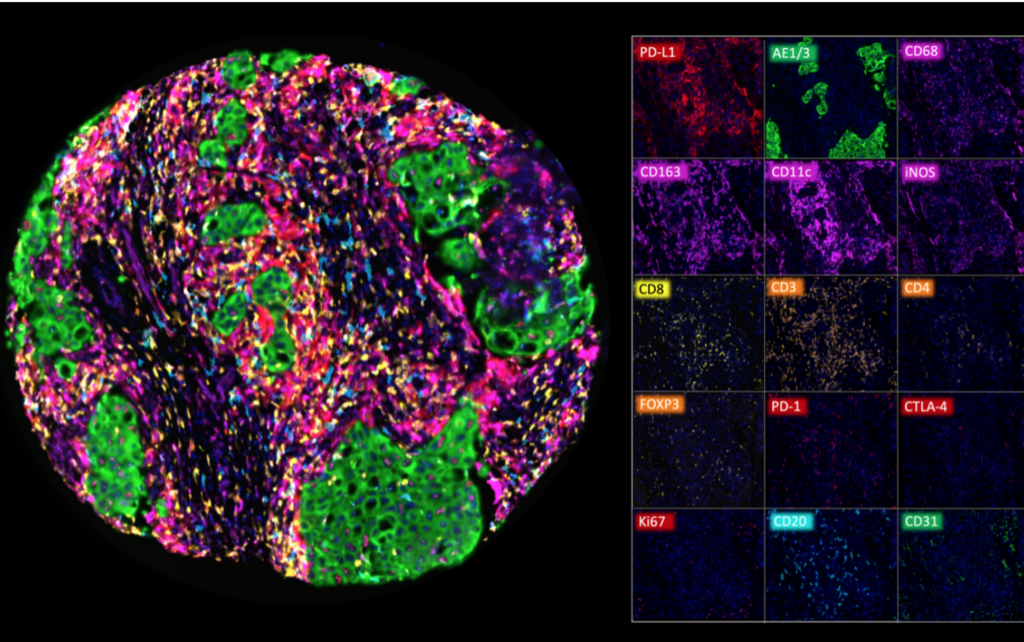
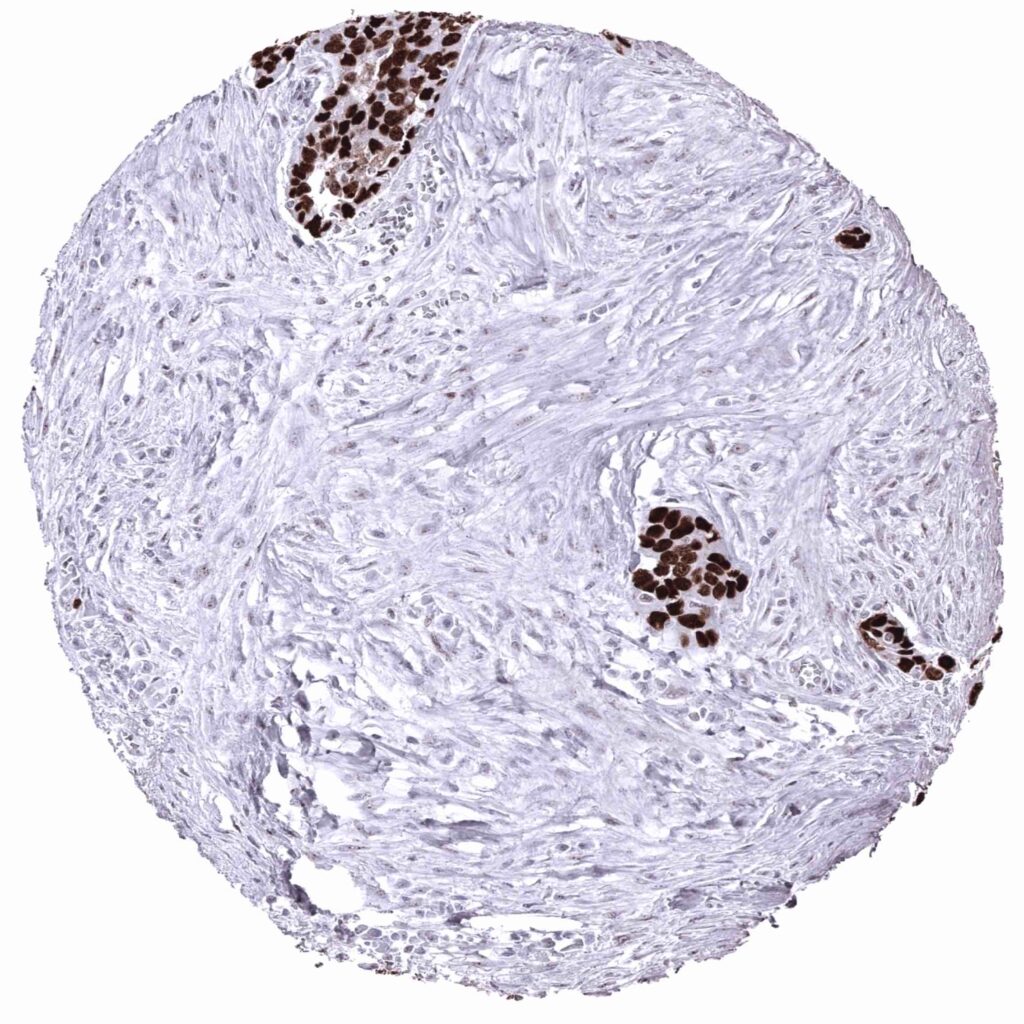
Mulitplex fluorescence IHC analysis of various immune cell types in a colorectal adenocarcinoma
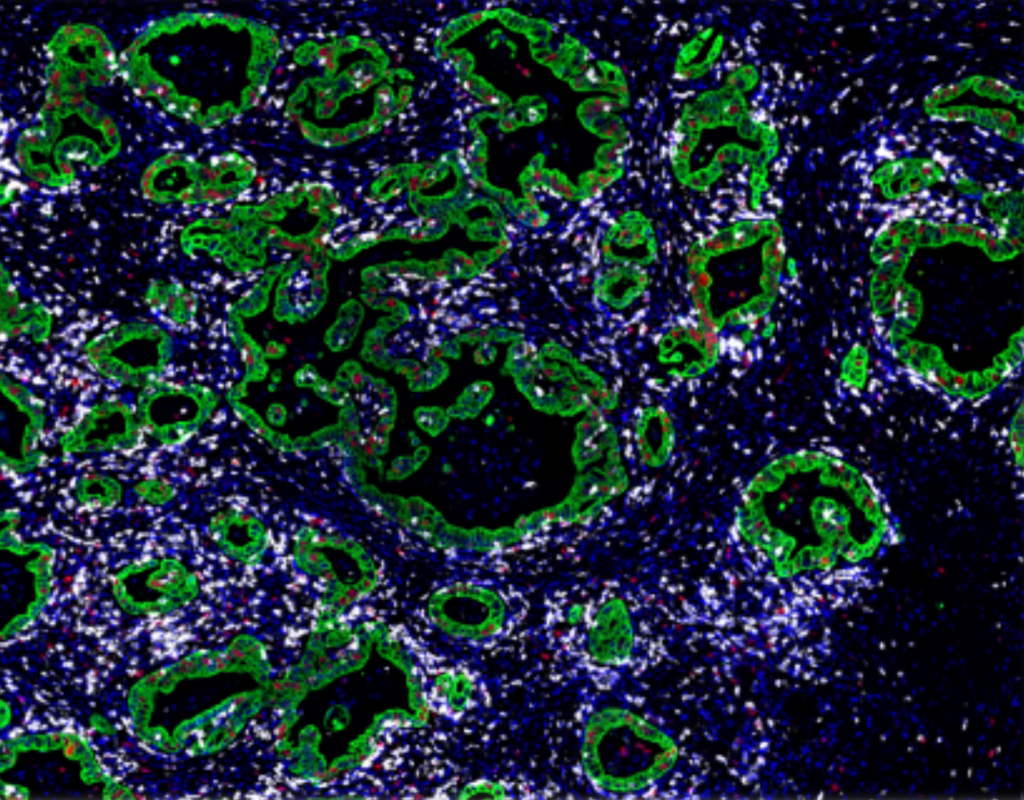
CD8+ cytotoxic T-cells (white) are adjacent to the colorectal cancer cell (green) that show a variable fraction of proliferating cells (Ki67, red)
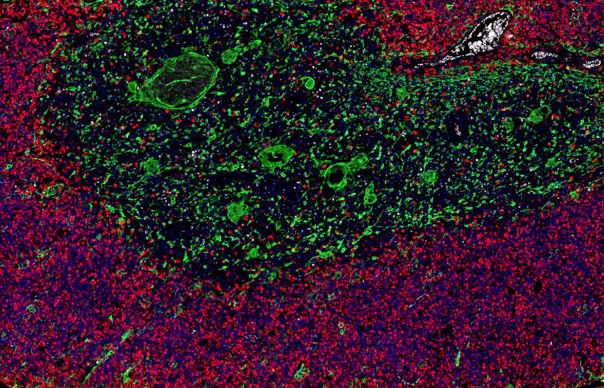
A high Ki67+ proliferation rate (red) is seen in cortical thymocytes. Thymic epithelial cells are shown in green (CKpan+)
A multiplex study using 7 MSVA antibodies (Bady et al. 2023)
Bady et al. “BLEACH&STAIN 15-marker Multiplexed Imaging in 3,098 Human Carcinomas Reveals Six Major PD-L1-driven Immune Phenotypes with Distinct Spatial Orchestration.” Molecular cancer research : MCR vol. 21,6 (2023): 605-613. doi:10.1158/1541-7786.MCR-22-0593
The following 7 MSVA antibodies were used in the study:
See also Table S2: Used antibodies
PD-L1
(MSVA-711R)
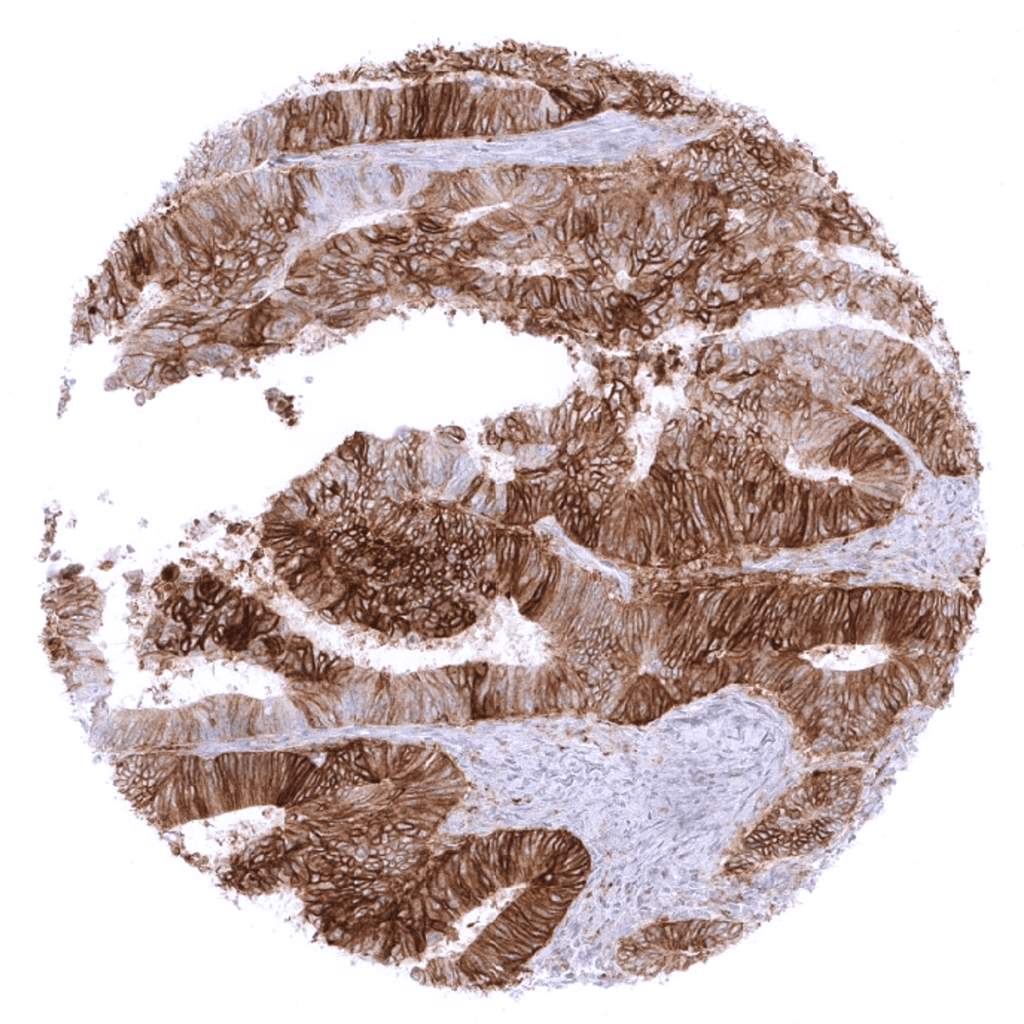
Patterns of PD-L1 immunostaining are highly heterogeneous in cancer tissues.
CTLA-4 / CD152
(MSVA-152R)
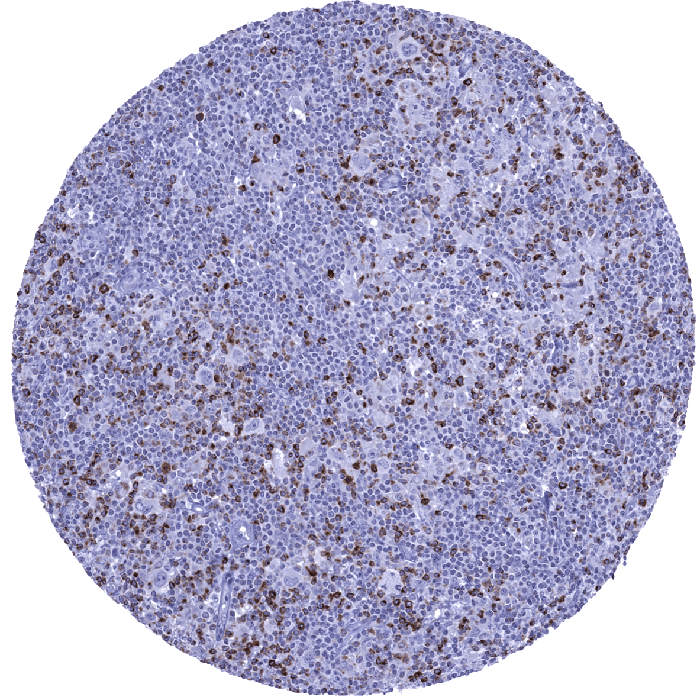
Pivotal target in immune-oncology (e.g. Hodgkin’s lymphoma). Suited for multicolor IF.
Cytokeratin, Pan
(MSVA-000R)

Antibody cocktail for the staining of as many as possible cells of epithelial origin.
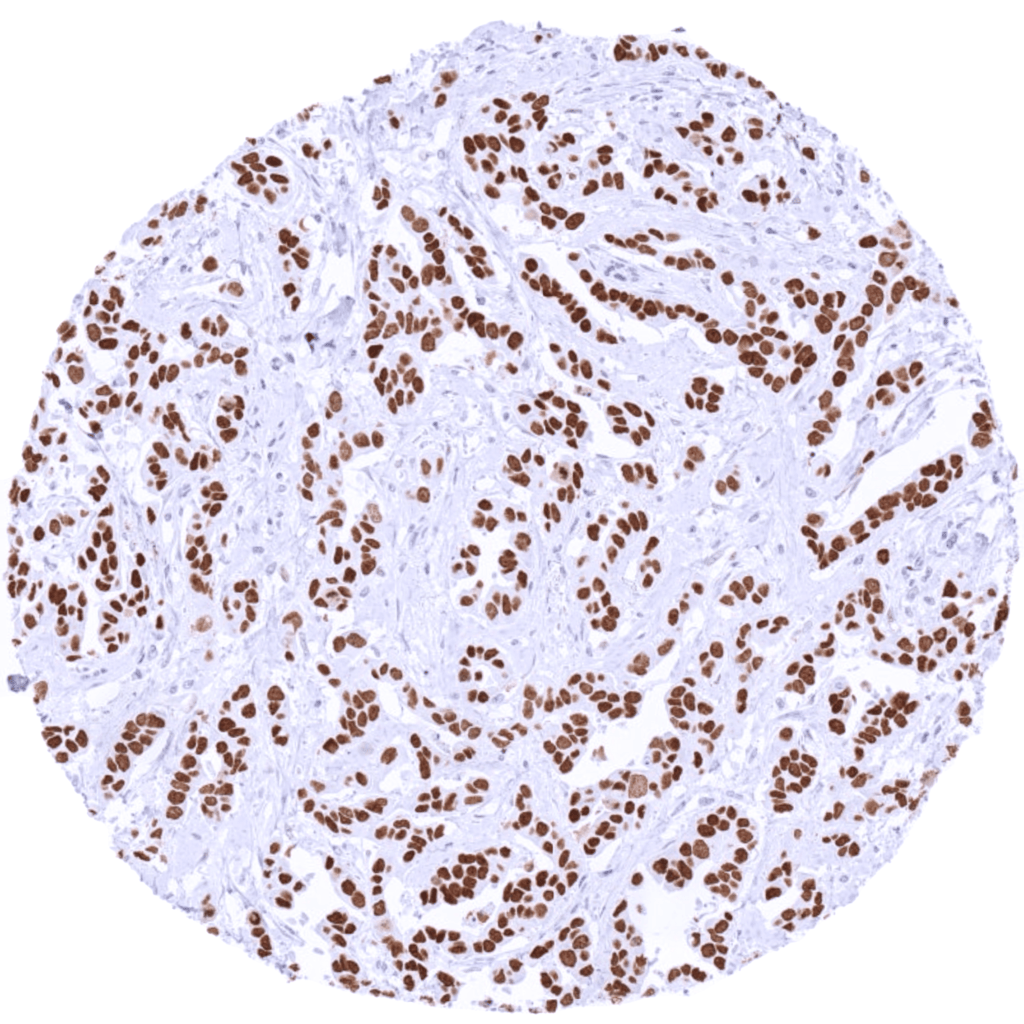
Ki-67
(MSVA-267M)
Ki-67 is a proliferation marker expressed by cells in G1, S, G2 and M phases of cell cycle.
CD-4
(MSVA-004R)
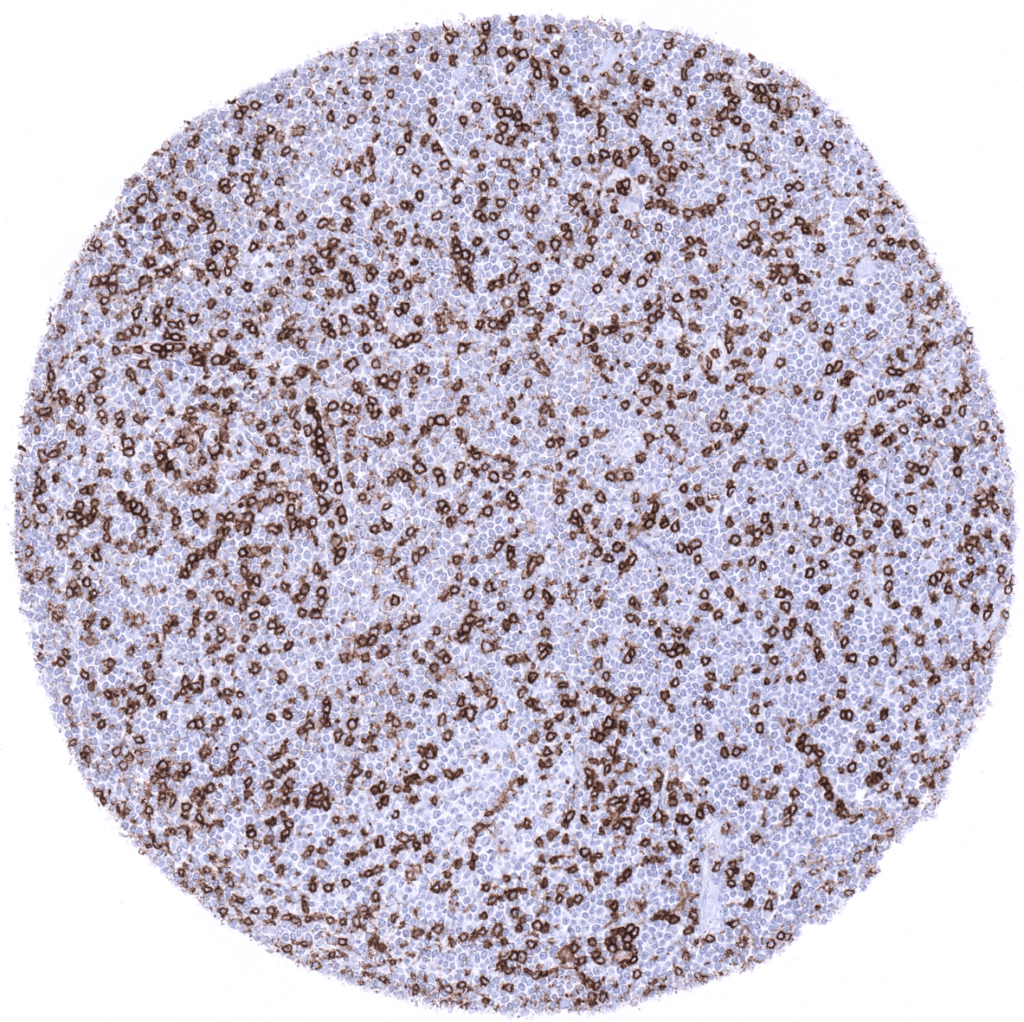
expressed on T helper lymphocytes.
CD31
(MSVA-031M)
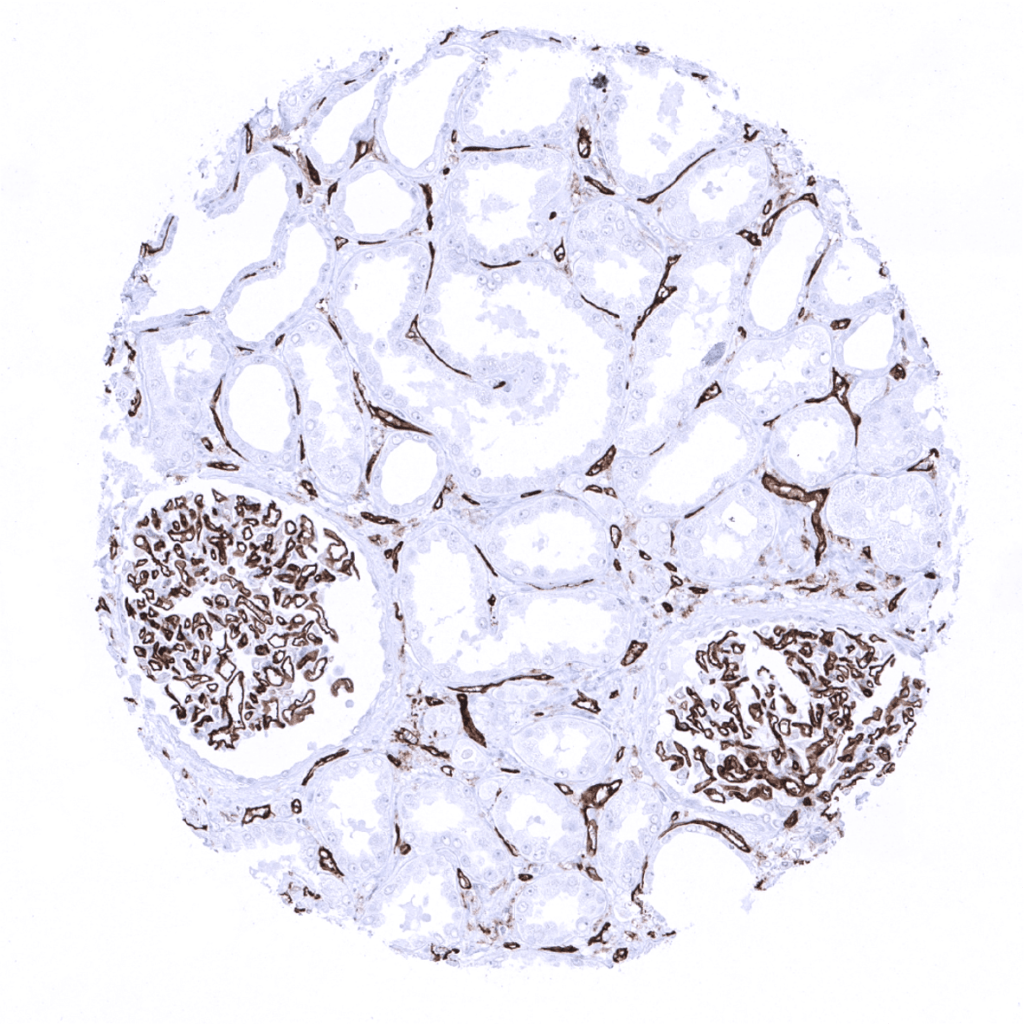
CD31 is expressed on endothelial cells.
CD20
(MSVA-020M)
CD20 is expressed on B lymphocytes.
Other MSVA validated antibodies to be highlighted
TIM-3
(MSVA-366R)
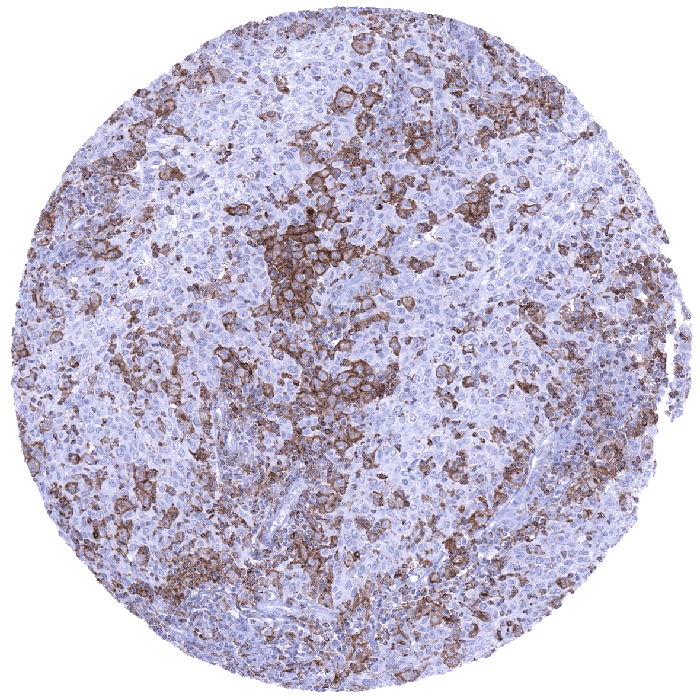
TIM-3 is an immune checkpoint that constitutes a “hot topic” in immune-oncology research.
CD45
(MSVA-045R)
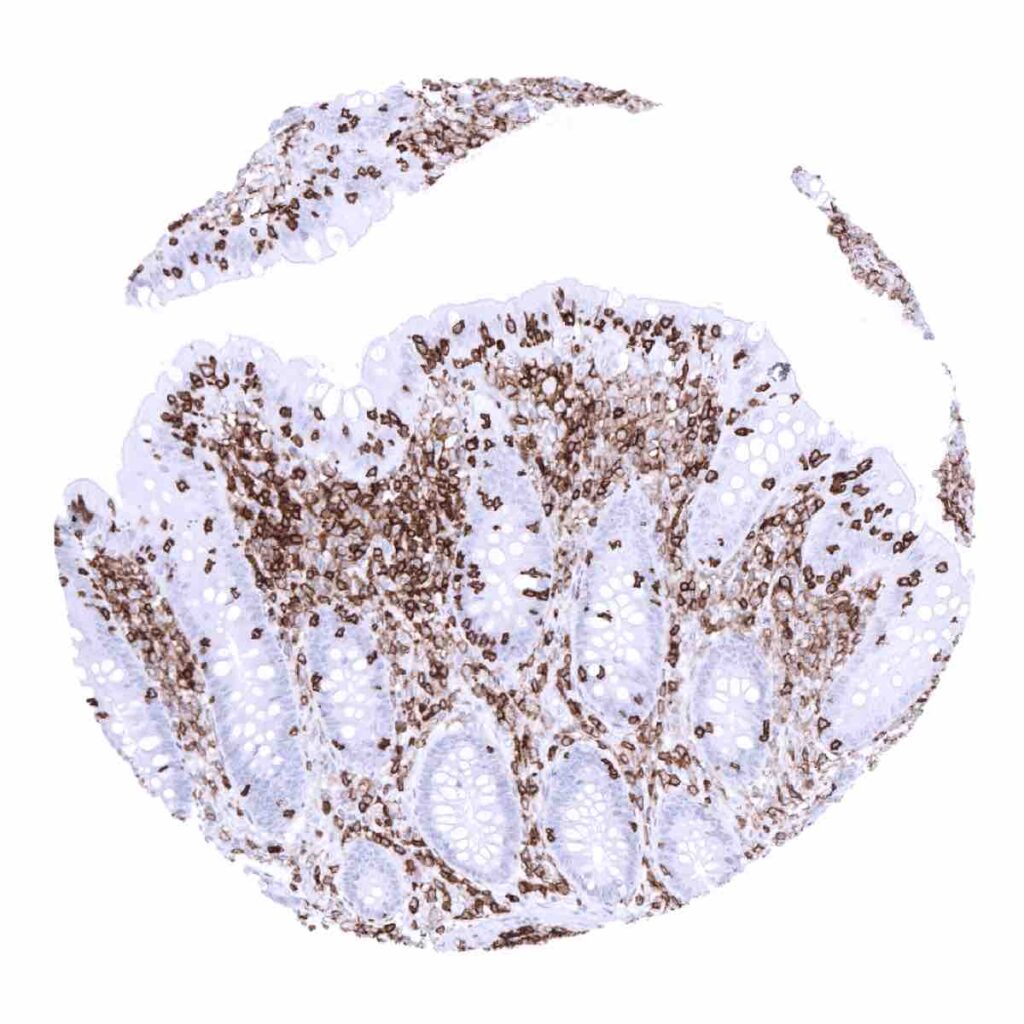
Expressed on hematolymphoid cells and in the vast majority of leukemias, malignant lymphomas and more
E-Cadherin
(MSVA-035R)
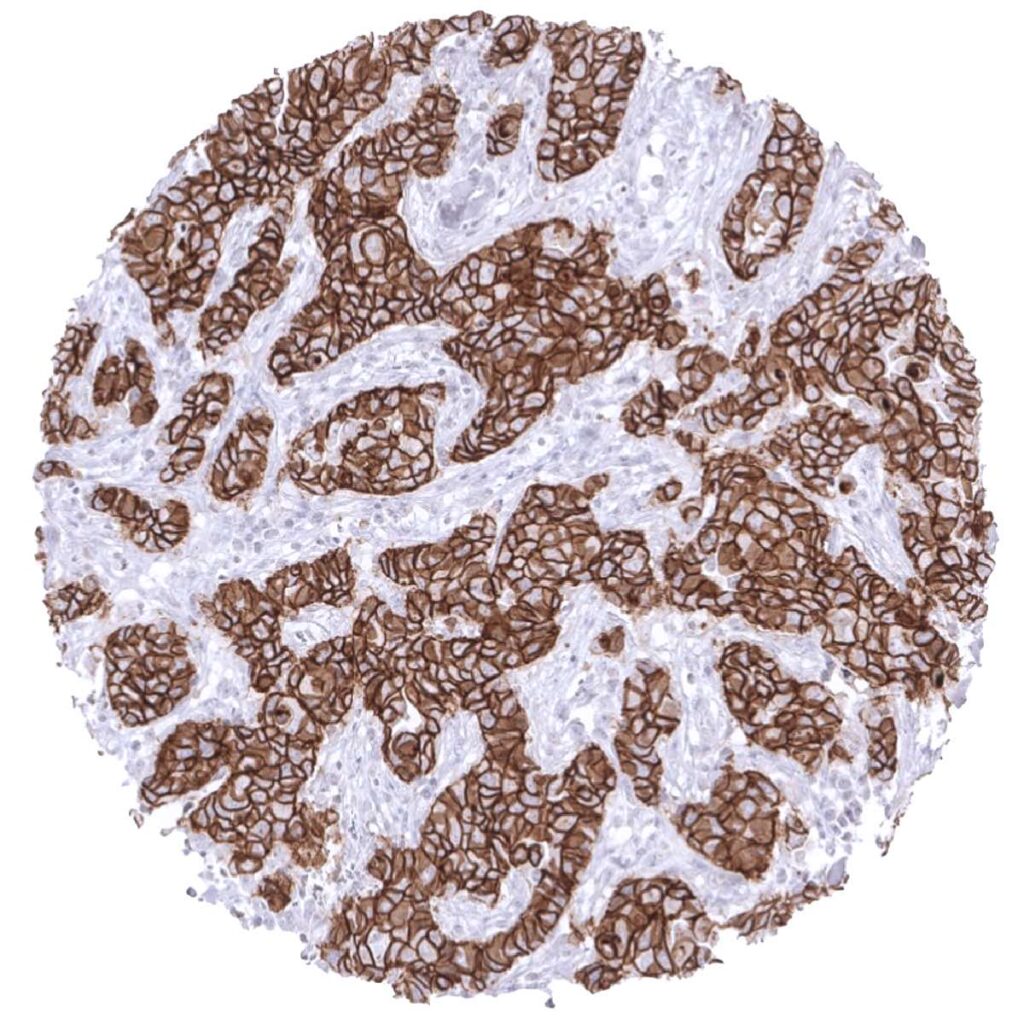
Important cell adhesion protein and therapeutic target. Loss of E-Cadherin expression is characteristic of lobular breast cancer.
PLAP
(MSVA-350R)
Marker for germ cell tumors and various types of adenocarcinomas.
Details
TACSTD2 / Trop-2
(MSVA-733R)
Trop-2 up-regulation has been reported from several cancer types.
Details