IBA-Lifesciences - Strep-tag® Technology
Cloning, expression, detection, purification, and immobilization of recombinant proteins
Excellent high-throughput possibilities!
IBA Lifesciences‘ Strep-tag® system enables cloning, expression, detection, purification, and immobilization of recombinant proteins. The highly specific interaction of the Strep-tag®II with Strep-Tactin® ensures efficient one-step purification of the protein of interest in unparalleled purity even from crude cell lysates. The mild and physiological conditions promote the yield of fully functional proteins, making the system particularly suitable for purification of enzymes as well as structural investigations, protein-protein interaction studies, ligand-receptor investigations, or even separation of living cells.
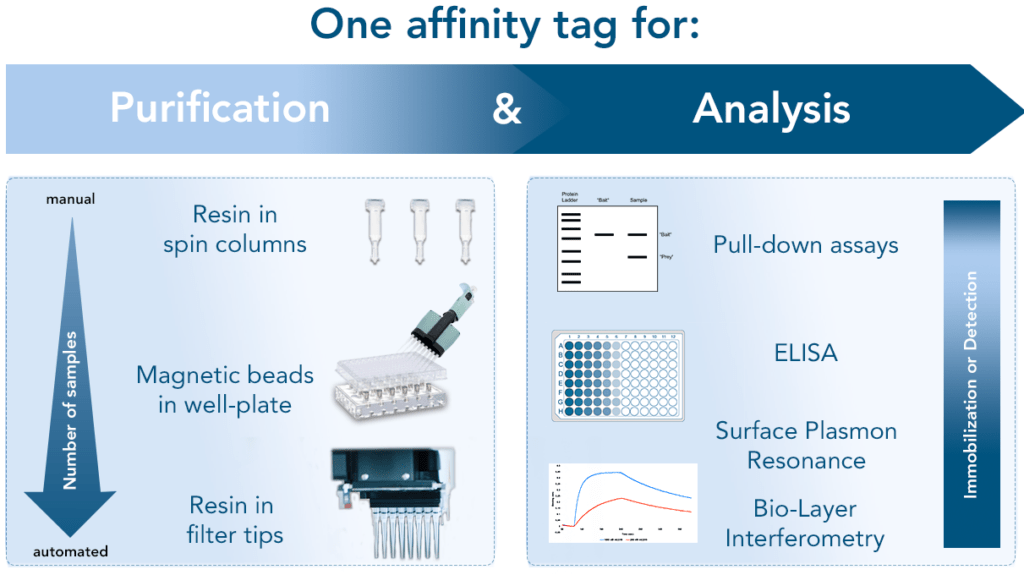
The Strep-tag® technology platform: From purification to analysis
High performance research tools for cell and protein isolation
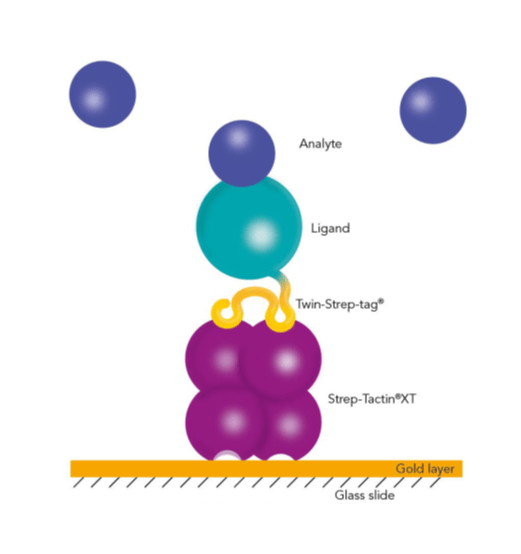
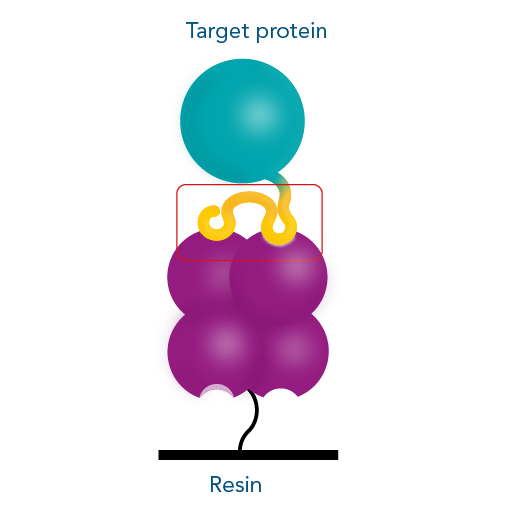
IBA Lifescience’s proprietary Strep-tag® technology exploits one of the strongest non-covalent interactions in nature: the interaction of biotin and streptavidin. The system is based on the highly selective and easily controllable interaction between the synthetic Strep-tag®II peptide and the specially engineered streptavidin, called Strep-Tactin®, which is one of the most stable proteins known. The Strep-tag®II binds specifically to the engineered streptavidins, Strep-Tactin® and Strep-Tactin®XT, by occupying the binding pocket of the natural ligand biotin. Hence, the interaction is easily reversible by excessive addition of the competitor.
The Strep-tag® technology is compatible with a large number of protein classes, e.g. metalloproteins, membrane proteins, and fragile protein complexes with multiple subunits. Simultaneously, a high tolerance towards different buffers and additives promotes its universal applicability. The near covalent affinity (pM range) of Twin-Strep-tag® to Strep-Tactin®XT expands the range of applications of the Strep-tag® system towards protein analysis, e.g. SPR analysis or bio-layer interferometry (BLI).
Strep-tag®: One tag, multiple applications
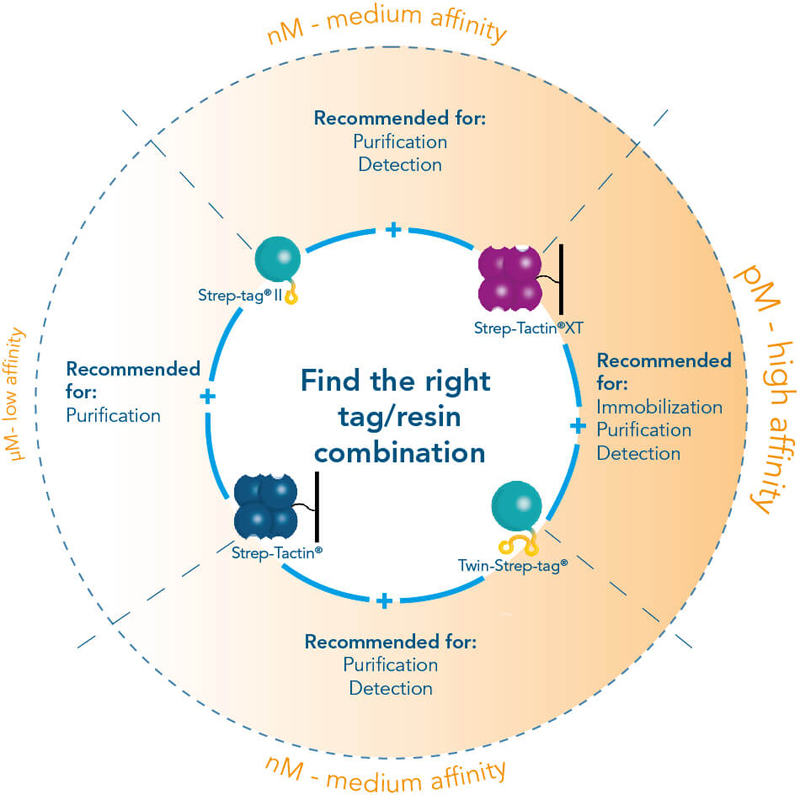
The Strep-tag® protein purification system comprises two affinity tags, the 8AA Strep-tag®II and its tandem version Twin-Strep-tag®. Both versions can bind to Strep-Tactin® and its high affinity variant Strep-Tactin® XT. Thereby the two tags differ in the affinities with which they bind. Depending on the application and properties of the protein of interest one can combine the different tags and Strep-Tactin® variants according to the required affinity.
The Strep-Tactin®XT – provides a binding affinity in low pM ranges in combination with Twin-Strep-tag® still maintaining binding reversibility and mild recovery of immobilized proteins. This improvement in affinity allows protein purification at high yields and purity, even for challenging proteins and from mammalian expression systems (e.g. Expi). Read more about the benefits of Strep-tag® purification in combination with high density mammalian protein expression in our white paper.
Furthermore, it fulfills the high demands of protein interaction analysis or assays and downstream applications like SPR, thus covering all steps from purification to immobilization efficiently.
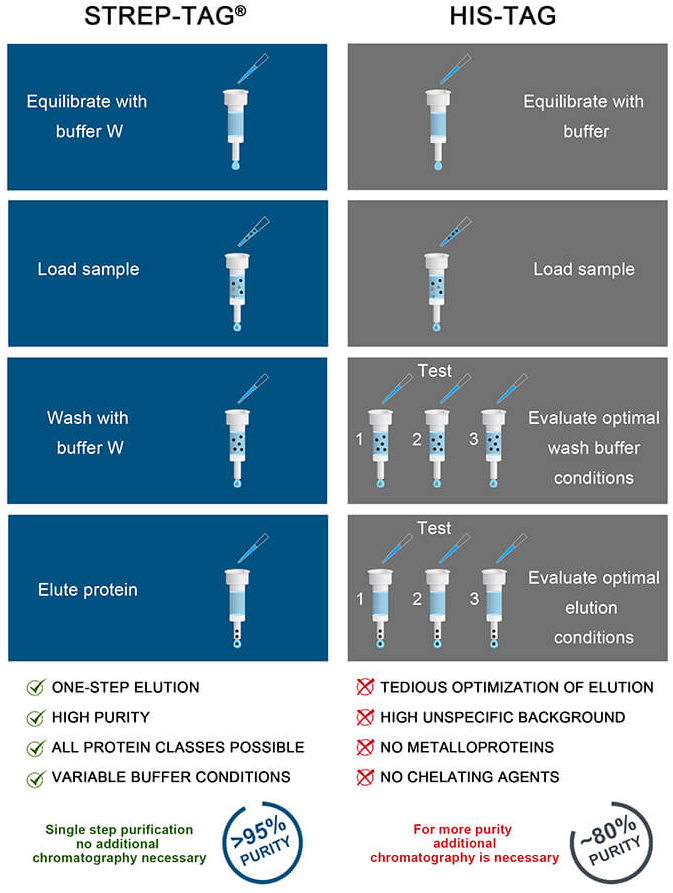
Benefits of Strep-tag® vs His-tag purification
Strep-tag® and His-tag are both widely used tools for affinity purification via a peptide tag. Choosing an appropriate affinity chromatography system for simple and efficient protein purification is a common question.
When it comes to purity and versatility in applications, some differences between both systems are obvious. The infographic on the right shows not only the time saving way of Strep-tag® protein purification but also the main key features of the Strep-tag® system at a glance.
Strep-tag® performs better than His-tag in purification of mammalian-expressed proteins
In combination with high density mammalian protein expression, the His-tag system can lead to poor purification results, if conditions are not optimized. In order to achieve pure proteins for downstream applications from the coupling of the His-tag system and high density mammalian systems, further adjustments like dialysis of the supernatant, lower media:resin ratios or the use of strip-resistant nickel resins become necessary. Such adaptions require additional optimization time and effort, however, these challenges can be avoided by using the Strep-tag® system.
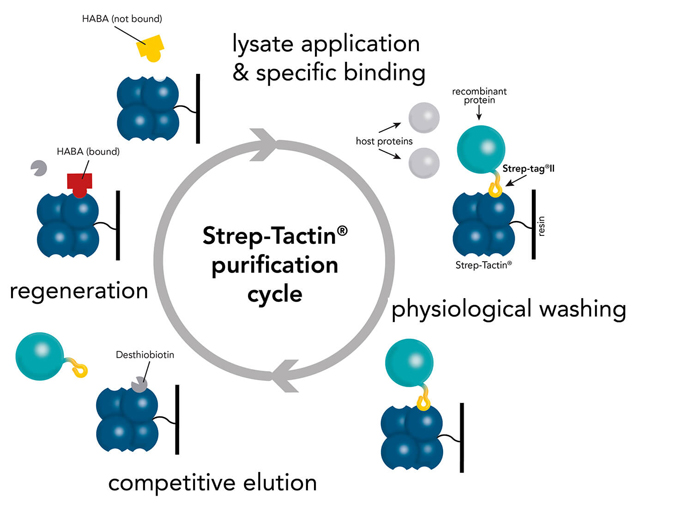
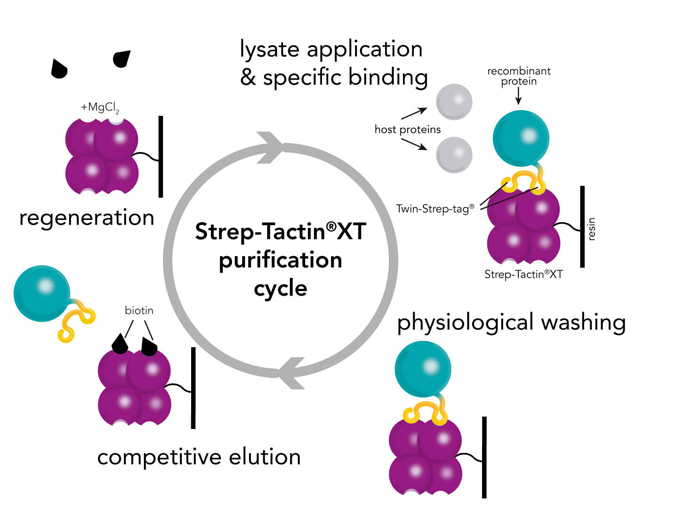
Strep-Tactin® and Strep-Tactin®XT purification cycle
Strep-Tactin® | Strep-Tactin®XT | |
|---|---|---|
Binding affinity | Strep-tag® II: μM range Twin-Strep-tag®: nM range | Strep-tag® II: nM range Twin-Strep-tag®: pM range |
Elution | Elution with Buffer E (2.5 mM Desthiobiotin) | Elution with Buffer BXT (50 mM Biotin) |
Regeneration | Elution with Buffer R (HABA) | Elution with 10 mM NaOH or 3 M MgCl2 |
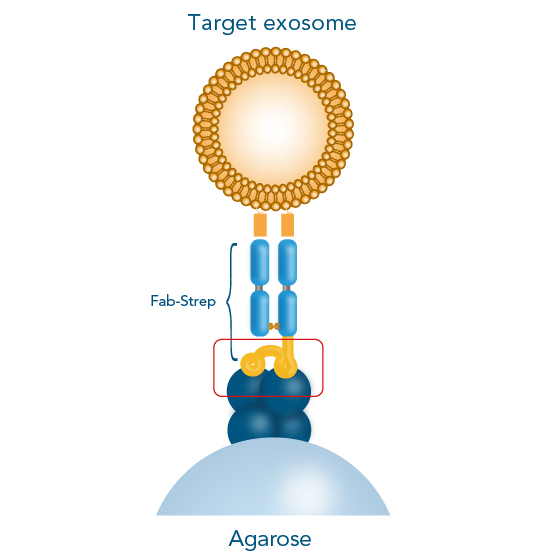
Protein-Protein Interaction
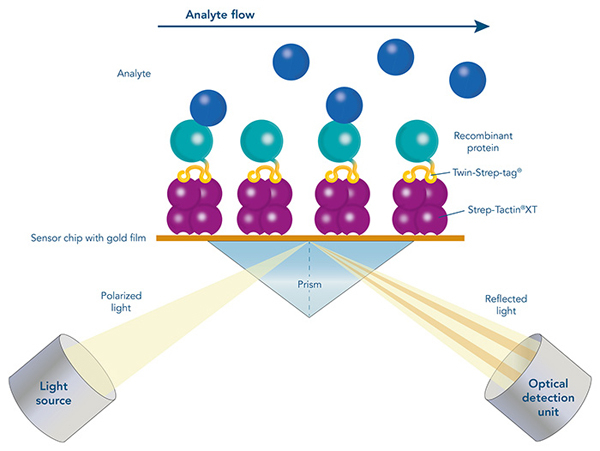
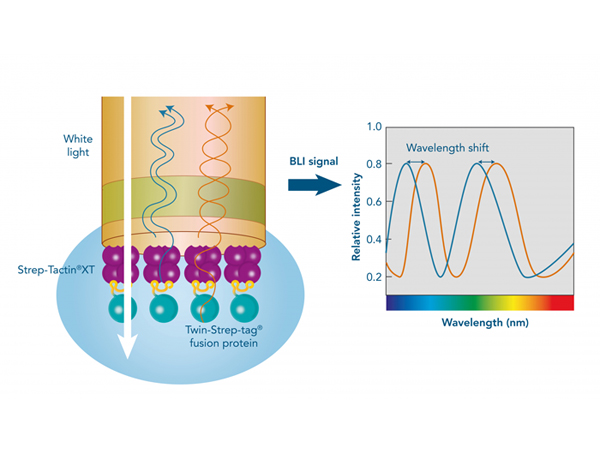
A crucial step in setting up an experiment to study protein-protein interactions by surface plasmon resonance (SPR), Bio-layer inferometry (BLI) or on the Biacore system is the immobilization of a protein to the sensor chip.
The Strep-tag® technology enables a directed, high-affinity immobilization that does not influence the activity of the ligand or require any modifications. Due to the high-affinity interaction and its high specificity, non-specific binding of host cell proteins is avoided, and ligands can be captured efficiently and directly from culture media. Moreover, Strep-Tactin®XT is compatible with many substances and the biosensors can be easily regenerated. The Twin-Strep-tag® Capture Kit provides all necessary products for the preparation of Strep-Tactin®XT-coated biosensor chips and the following measurement