IMCS - Automated high-throughput purifications in pipette tips
IMCStips | IMCSzyme - patented purification solutions for proteomics and genomics
IMCStips® is a patented, automated high-throughput dispersive solid-phase extraction (dSPE) technology used in proteomics and genomics. The loose resins contained in the pipette tips mix with sample solutions during aspirate and dispense cycles, ensuring maximum contact between the resin and your analytes of interest. This results in higher binding capacities and consistent, reproducible results in a short time.
IMCSzyme® is a collection of genetically modified pure beta-glucuronidase products with high efficiency. Consistent, stable and pure enzymes.
IMCStips is perfect for automating protein and nucleic acid purification
IMCStips® are the definitive choice when it comes to automated sample preparation. Designed with efficiency and user-friendliness in mind, this product uses patented dispersive solid-phase extraction (dSPE) to automate workflows while enhancing accuracy and consistency.
IMCStips are compatible with a variety of automated liquid handling platforms, including Hamilton, Dynamic Devices, and INTEGRA VIAFLO 96. Purify and isolate samples in as little as 30 minutes!

- Consistent, high recoveries
- Higher binding capacities
- Reproducible results in a short time
- Flexible sample volumes
- Customized applications
- Streamlined, automated workflows for sample preparation, including protein purification, His-tag purification, nucleic acid purification, etc.
IMCStips® containing Strep-Tactin®XT resin (IBA Lifesciences) are available. View application note.
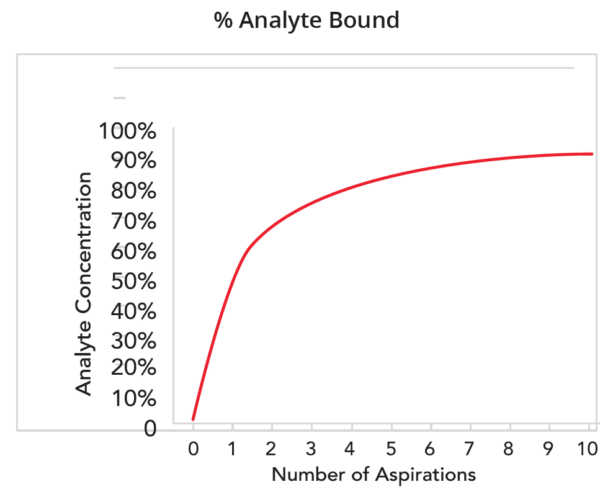
Analyte binding per aspiration was assessed using bromophenol blue dye diluted in water at varying concentrations. The binding capacity of IMCStips is limited by concentration as well as the chemical property of the analyte of interest (i.e logP values).

Purification of a single log of CHO cell fermentation using MabSelect SuRe LX resin (50 μL resin bed) by centrifugation (spin) and IMCStips. For consistency, the spin method utilized 100 x g RCF for five minutes and flow through samples were re-applied to the column either 3x or 5x, similar to the tip format. Spin format consistency yielded less mAb than tip based format, as indicated by the UV-Vis measurements (Abs @ 280 nm) above.
Resins and Applications of IMCStips
Affinity Purification
Resin Options
- Protein A, G, A/G
- Ni-IMAC
- Streptavidin
- Strep-Tactin
- Peptide-based
Applications
- Antibody purification
- Recombinant protein purification
- Biotinylated protein purification
- Purification of Strep-tagged proteins
Size Exclusion
Resin Options
- SizeX100
- SizeX150
Applications
- Buffer exchange
- Size exclusion chromatography
- Automated multi-attribute method (MAM)
Nucleic
Acid
Resin Options
- µPure LE
- µPure LE Kit
- µPure LE Kit with SizeX
- IMCStips for PCR Cleanup
Applications
- Nucleic acid purification or extraction
- Low endotoxin plasmid purification
- PCR cleanup or PCR product purification
- NGS library prep or cleanup
Phospho-peptide
Resin Options
- PolyTi™
- Zirconia
Applications
- Automated phosphopeptide enrichment
Ion Exchange
Resin Options
- µSAX
- Macro SCX
- Macro SAX
- Macro WAX
- Macro WCX
Applications
- Ion exchange chromatography
- µPure LE: Automated low endotoxin plasmid purification
Reverse Phase
Resin Options
- P
- Large Pore C4
- Silica C8
- Silica C18
- OligoR
Applications
- Peptide desalting
- Protein desalting
- DMT-on oligonucleotide purification
Automated Purification of IgG Antibodies using Protein A IMCStips®
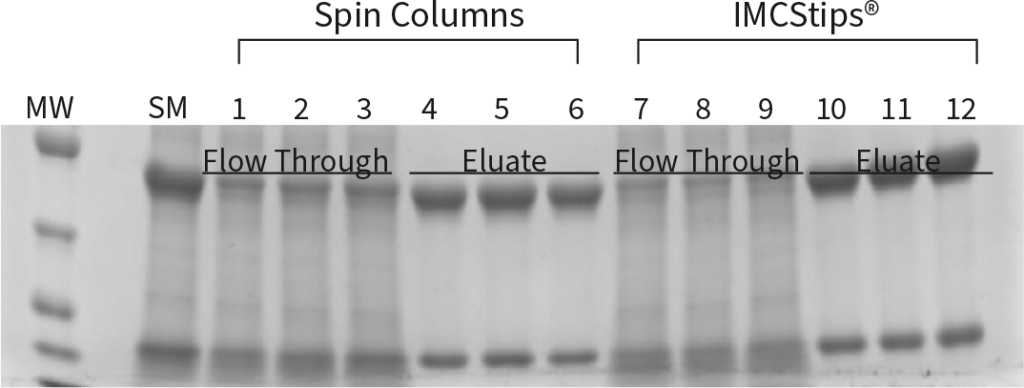

Purification of a single lot of CHO cell fermentation using Protein A resin (50 µL resin bed) by centrifugation (spin) and IMCStips. For consistency, the spin method utilized 100 ×g for five minutes and flow through samples were re-applied to the column either 3x or 5x, similar to the tip format. The spin columns yielded less IgG than IMCStips, as indicated by both UV-Vis measurements and by SDS-PAGE.
Automated Affinity Purification and Buffer Exchange with IMCStips®

(A) Histidine labeled proteins (GFP, PaS, GusA) were added into bacterial lysates and purified with 15 µL resin Ni-IMAC IMCStips followed by buffer exchange with SizeX IMCStips.

(B) The recoveries for the Ni-IMAC IMCStips was optimal for 100 and 200 µg protein loads due to the 15 µL resin bed. More resin will assist with higher recoveries for larger quantities (400 and 600 µg).

(C) SDS PAGE of the proteins at different quantities match the Bradford quantifications (see graphs in A).

(D) Purified PaS are active (red wells) after undergoing affinity purification followed by buffer exchange on the liquid handler, and there is no detectable carryover between wells or tips.



